Neurodegenerative diseases: Highly responsive immune cells seem to be beneficial for the brain
14 Feb 2022
New insights into the mechanisms of neurodegenerative diseases
14 Feb 2022
New insights into the mechanisms of neurodegenerative diseases
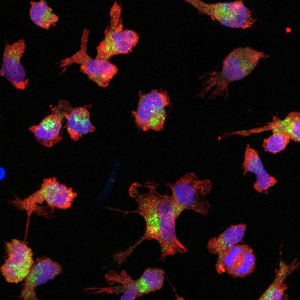
Microglia derived from stem cells. Source: LMU Klinikum / Dominik Paquet, Sophie Robinson
Findings by researchers from Germany support the view that hyperactive immune cells in the brain can have a protective effect in the course of neurodegenerative diseases. Experts from Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), LMU and LMU Klinikum report on this in the scientific magazine “The EMBO Journal”. The scientists are currently considering that modulating the activity of immune cells in the brain via a receptor called TREM2 may significantly impact neurodegenerative disease processes. Thus, they see activating TREM2 as a promising approach for drug research.
The immune cells of the brain – called “microglia” – act against pathogens, help to clear up cellular debris and also maintain neuronal health. However, in Alzheimer’s disease and other neurodegenerative diseases, these cells enter a “hyperactive” state, traditionally considered an excessive immune response because it is associated with chronic and thus harmful inflammatory processes.
However, the current results put this view partly into a new perspective. “Contrary to common belief, our findings support the hypothesis that hyperactive microglia have their good side. Recently, there has already been some evidence for this. Our study now provides further indications,” says Professor Christian Haass, a research group leader at DZNE, Chair of Metabolic Biochemistry at LMU and member of the Cluster of Excellence SyNergy.
In previous studies, Haass and colleagues had identified a protein called TREM2, which is anchored in the cell membrane of microglia, as an “activity switch”. Using antibodies that bind and activate TREM2– co-developed with the U.S. company Denali Therapeutics – the researchers succeeded in toggling this molecular switch, thereby boosting microglial activity. “At the time, we saw in laboratory experiments that microglia activated in this way more effectively eliminate the protein deposits typical of Alzheimer’s disease, the notorious amyloid plaques,” explains Haass. “However, we were concerned that too much activation of microglia could cause harm, as is commonly believed.”
The current studies expand upon the investigations carried out back then. Instead of increasing microglia activity, the researchers now pursued the opposite. “We wanted to know the impact on disease pathology when we downregulated the activity of hyperactive microglia,” Haass says. This time, an antibody was used that disabled the TREM2 receptor and thus reduced the activity of the immune cells in the brain.
As an example of a neurodegenerative disease, the researchers focused on “GRN-associated frontotemporal lobar degeneration”, GRN-FTLD for short. “This is a genetic, rare form of dementia that comes with a wide range of abnormal behaviors. Some of the affected individuals are impulsive and aggressive, while others are apathetic,” explains Professor Dominik Paquet, a neurobiologist at the Institute for Stroke and Dementia Research at LMU Klinikum, whose research group was also involved in the current study.
“GRN-FTLD is well-described and there are good options for laboratory studies. Therefore, we used this disease as an example to investigate how hyperactive microglia contribute to the pathology of neurodegeneration,” says Dr. Anja Capell, biochemist at LMU München, who co-designed the current study.
The research team used different cell cultures for their experiments. These included either microglia derived from human stem cells or cells obtained directly from patients with GRN-FTLD. Mice with genetic traits characteristic of GRN-FTLD were also studied.
“Our data suggest that it is indeed possible to diminish microglia activation state via inhibition of TREM2 signaling. Hyperactivity is therefore reversible and not a one-way street, which is not a given,” says Anja Capell. “However, the pathology was not improved but worsened as a result; loss of contacts between neurons, the synapses, increased. We also found that the level of a biomarker for neuronal damage rose.”
These results are unexpected. “We were surprised ourselves. But contrary to common belief, hyperactivated microglia seem to retain certain neuroprotective functions. At least this applies to the model system we studied,” says Christian Haass. “Conversely, this means that a controlled increase in the activity of microglia could help to contain the disease process to some extent. For this, I consider targeting the TREM2 receptor with an agonist antibody, that’s an activating antibody, to be promising. We intend to pursue this idea further.”
Anika Reifschneider et al.: Loss of TREM2 rescues hyperactivation of microglia, but not lysosomal deficits and neurotoxicity in models of progranulin deficiency. The EMBO Journal 2022