The key to the lock gates
9 Feb 2017
Ion channels control the passage of charged atoms into and out of cells to regulate vital cellular processes. In some cases, adaptor proteins control their gating mechanism.
9 Feb 2017
Ion channels control the passage of charged atoms into and out of cells to regulate vital cellular processes. In some cases, adaptor proteins control their gating mechanism.
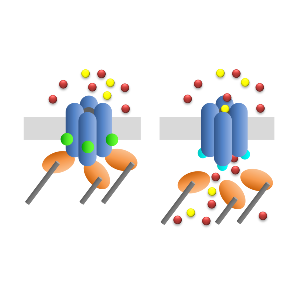
Channel proteins (blue), adapter proteins (orange), PIP2 (green) and diacylglycerol (turquoise) are involved in the activation.
Ion channels are proteins that form pores in cellular membranes, which can be opened and shut like lock gates to allow the passage of electrically charged atoms (ions). Members of this class of proteins are crucial components involved in a wide range of processes that are essential for survival. In order to ensure that they correctly perform these functions, however, the opening and closing of these pores must be carefully regulated. LMU researchers led by Professor Dr. Michael Mederos y Schnitzler and Dr. Ursula Storch at the Walter Straub Institute for Pharmacology and Toxicology at LMU have now uncovered an activation mechanism in which an accessory molecular adaptor acts as a fail-safe mechanism to prevent inappropriate opening of two related ion channels. Their results have now been published in the journal PNAS.
The study focused on the ion channels TRPC4 und TRPC5, which belong to a particular family of cation channels. TRPC4 and 5 are found in a variety of cell types and tissues and, when activated, they selectively permit the passage of sodium (Na+) and calcium (Ca++) cations across cellular membranes. But how the two are activated has been unclear up to now. “The evidence available has been contradictory,” says Mederos y Schnitzler. But he and his colleagues have now conclusively shown that TRPC4 and 5 are maintained in the inactive state by the binding of specific adaptor proteins, together with a small lipid molecule called PIP2. In both cases, enzymatic cleavage of PIP2 results in a specific change in the structural conformation of the channel, which in turn leads to dissociation of the adaptor. This process exposes a binding site on the TRPC protein for the intracellular messenger diacylglycerol (DAG), and attachment of DAG activates the channel, allowing cations to permeate though the pore.
The new findings clearly demonstrate that, contrary to what had been previously believed, both TRPC4 and TRPC5 are in fact sensitive to DAG. However, unlike all other members of the TRPC family of ion channels, TRPC4 and 5 cannot be activated directly by DAG, because their adaptor proteins dynamically regulate its access to the proteins, subsequent to the cleavage of PIP2. This is a novel role for an adaptor, as most serve as passive scaffolds for the assembly of protein complexes.
Depending on their subcellular localization, the adaptors that interact with TRPC4 and TRPC5 can promote or inhibit tumor cell growth, and since TRPC4 and 5 are expressed in many types of tumor cells, their interactions are also of medical relevance. This makes ion-channel/adaptor-protein complexes possible targets for anti-cancer drugs.