How Roquin controls the activity of immune cells
23 Nov 2021
LMU immunologists have discovered how mutations in Roquin-1 trigger autoimmunity, but can also improve the body’s fight against cancer cells.
23 Nov 2021
LMU immunologists have discovered how mutations in Roquin-1 trigger autoimmunity, but can also improve the body’s fight against cancer cells.
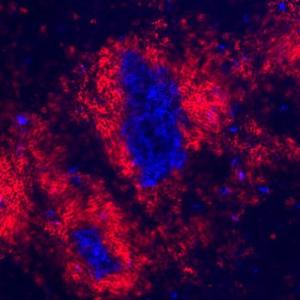
Spontaneous formation of germinal centers in the spleen of genetically modified mice, a sign of the development of autoimmunity. | © Heissmeyer Group
With autoimmune diseases such as lupus erythematosus, severe inflammation occurs in different areas of the organism. The immune system mistakenly identifies the body’s own structures as foreign and attacks them. Such disorders have various triggers, and only a handful of known mutations in individual genes lead to autoimmunity. These include the gene that codes for Roquin-1. The so-called sanroque mutation induces a lupus-like syndrome in mice.
“Such mutations teach us how our body protects itself against autoaggressive reactions of the immune system,” explains Professor Vigo Heissmeyer, who is a researcher at the Institute for Immunology at LMU and the Molecular Immune Regulation Research Unit at Helmholtz Zentrum München. By means of functional investigations and mouse models, he and his team have now shown how the exchange of a single amino acid — such as the sanroque mutation in Roquin-1 — leads to stronger autoimmunity. “We think we’ve found a target structure that controls autoimmunity and which could even be suitable for enhancing anti-tumor responses,” says Heissmeyer, outlining the key results of the experiments from his team.
Together with colleagues from the Helmholtz Zentrum München and LMU, he had previously elucidated molecular functions of Roquin-1. The protein plays a key role in the adaptive immune response by controlling the activation and differentiation of T cells via the regulation of gene expression. Interestingly, it had been suggested that the Regnase-1 protein works in the same way. “What we didn’t understand before was why the exchange of an amino acid in the sanroque mutation of Roquin-1 leads to a very similar form of autoimmunity as the loss of the gene encoding Regnase-1,” says Heissmeyer.
The research group has now been able to demonstrate that Roquin-1 binds directly to the Regnase-1 protein so as to efficiently control the expression of certain genes. Surprisingly, the amino acids involved in this binding were discovered to be in close spatial proximity to the amino acid that was altered in the sanroque mutant. This suggested that they represent an extended binding site. In the gene encoding Roquin-1 in mice, the researchers successfully used CRISPR-Cas technology to replace individual amino acids that are involved in the binding to Regnase-1 with other specific amino acids. During the protein biosynthesis, this produced Roquin-1 proteins that interacted much more weakly with Regnase-1. These novel mutations led to autoimmunity in the rodents.“ Our data shows that the physical interaction of Roquin-1 with Regnase-1 is of key importance when it comes to controlling the activity of immune cells,” summarizes the LMU scientist.
Although the observed autoimmunity damages the organism and leads to illnesses, there could be benefits for cancer patients in an enhanced activation of immune cells that fight tumors. “Mechanisms in T cells that our immune system has developed to prevent autoimmunity are actually used by the tumor to silence T cells,” explains Heissmeyer. Accordingly, mice with the Roquin-1 gene mutations described above produced T cells that attacked malign cells with greater vigor after transfer into tumor-bearing mice.
This makes Roquin-1 an interesting target structure for oncology. Future research projects could seek to develop an inhibitor that reduces interactions between Roquin-1 and Regnase-1 — and that activates immune cells. “We expect that this will give a strong boost to the T cell response against tumors for a limited period of time,” says Heissmeyer.
Gesine Behrens et al: Disrupting Roquin-1 interaction with Regnase-1 induces autoimmunity and enhances anti-tumor responses. Nature Immunology, 2021.